Vaccines have been one of the greatest achievements in modern medicine, playing a crucial role in preventing infectious diseases and saving millions of lives. Over the years, the process of developing, testing, and distributing vaccines has evolved significantly. Advances in science and technology have made vaccines more effective and easier to deliver, but challenges remain in ensuring global access and addressing public health concerns. This article takes you through the journey of vaccines, from their early development to their current distribution systems, and highlights the future potential of vaccination in global health.
The History of Vaccines: A Groundbreaking Discovery
The story of vaccines begins in the late 18th century when Edward Jenner discovered the first vaccine. Jenner observed that milkmaids who contracted cowpox appeared to be immune to smallpox, a deadly disease. In 1796, he tested his hypothesis by inoculating a young boy with cowpox, and later exposing him to smallpox. The boy did not contract the disease, marking the birth of vaccination.
Over the next century, vaccines were developed to combat various infectious diseases, including rabies, cholera, and tuberculosis. However, it wasn’t until the mid-20th century, with the advent of more advanced technologies, that vaccine development accelerated, leading to the creation of vaccines for diseases like polio, measles, and influenza.
The Vaccine Development Process: From Concept to Approval
The journey from the initial idea for a vaccine to its widespread distribution involves several critical stages. These stages ensure that vaccines are safe, effective, and ready for mass use.
1. Research and Discovery
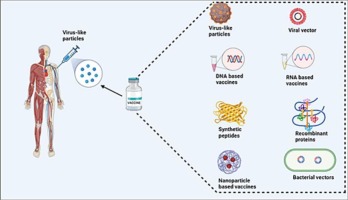
Vaccine development begins with understanding the pathogen (virus or bacteria) that causes a disease. Researchers identify the parts of the pathogen—such as proteins—that trigger an immune response in the body. These components are then used to create the vaccine.
In modern vaccine research, scientists use various approaches:
- Inactivated or killed viruses: These vaccines use pathogens that are killed or inactivated, so they cannot cause disease.
- Live attenuated vaccines: These vaccines use weakened forms of the virus or bacteria that still trigger an immune response without causing illness.
- Subunit, recombinant, or conjugate vaccines: These vaccines use only parts of the pathogen, such as proteins or sugars, to stimulate immunity.
Advances in genetic engineering, such as mRNA technology, have also allowed for faster development, as seen in the rapid creation of COVID-19 vaccines.
2. Preclinical Testing
Before human trials, vaccines undergo preclinical testing, where they are evaluated in laboratory settings using animal models. This stage helps assess the vaccine’s safety and ability to provoke an immune response.
3. Clinical Trials
Once a vaccine passes preclinical testing, it moves into clinical trials, which are conducted in three phases:
- Phase 1: Small-scale testing involving healthy volunteers to assess the vaccine’s safety, dosage, and immune response.
- Phase 2: Larger groups of people are tested to further evaluate safety and identify potential side effects. This phase also begins to assess the vaccine’s efficacy.
- Phase 3: The vaccine is tested in a large population to confirm its effectiveness and safety. This phase typically involves thousands of volunteers and can take several years to complete.
4. Regulatory Approval
Once the clinical trials are completed, the vaccine manufacturer submits the data to regulatory authorities, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), for approval. These agencies rigorously evaluate the safety and efficacy data before granting approval for the vaccine’s use in the general population.
5. Post-Market Surveillance
Even after a vaccine is approved, its safety continues to be monitored through post-market surveillance. This helps identify rare side effects that may not have appeared during clinical trials, ensuring that the vaccine remains safe for public use.
The Role of Technology in Vaccine Development
Technology has played a pivotal role in advancing vaccine development in recent years. Innovations such as mRNA technology, DNA vaccines, and improved diagnostic tools have accelerated the speed at which vaccines are developed, tested, and distributed.
- mRNA Vaccines: The COVID-19 pandemic highlighted the potential of mRNA technology, which enables the rapid development of vaccines. Unlike traditional vaccines, mRNA vaccines use a small piece of the pathogen’s genetic material to instruct cells to produce a protein that triggers an immune response.
- AI and Data Analytics: Artificial intelligence (AI) and data analytics help researchers identify potential vaccine candidates faster. AI algorithms analyze vast amounts of data to predict how pathogens might evolve and which vaccine components will be most effective.
- Genomic Sequencing: Advances in genomic sequencing have allowed scientists to study the genetic makeup of pathogens more quickly and accurately, speeding up the vaccine development process.
Distribution: Getting Vaccines to the Masses
Once a vaccine is developed and approved, the next challenge is ensuring its widespread distribution. The logistics of vaccine distribution are complex and require careful planning to ensure that vaccines reach every part of the world efficiently.
1. Cold Chain Requirements
Many vaccines, particularly those involving mRNA technology, need to be stored at very low temperatures, often referred to as the “cold chain.” This adds significant logistical challenges, especially in regions with limited infrastructure or in low-income countries. Solutions such as ultra-cold freezers and temperature-monitoring technologies have been developed to address these challenges.
2. Global Vaccination Campaigns
Global initiatives, such as GAVI, the Vaccine Alliance, and the World Health Organization (WHO), work to ensure that vaccines are distributed fairly and equitably around the world. These organizations provide funding, resources, and logistical support to help ensure vaccines reach underserved populations.
Efforts to ensure equitable vaccine distribution became particularly critical during the COVID-19 pandemic, as countries with fewer resources struggled to secure enough vaccines. Partnerships between governments, pharmaceutical companies, and international organizations have played a key role in ensuring that vaccines are delivered to all corners of the globe.
3. Public Health Infrastructure
An effective vaccination campaign relies on strong public health infrastructure. This includes vaccination sites, trained healthcare workers, and effective communication strategies to educate the public about the benefits of vaccination and address any concerns or misconceptions.
Challenges in Vaccine Distribution and Access
Despite the advancements in vaccine development and distribution, several challenges remain in ensuring that everyone has access to life-saving vaccines:
- Vaccine Hesitancy: Misinformation and distrust of vaccines can lead to vaccine hesitancy, which hampers vaccination efforts. Public health campaigns are essential in educating the public about the safety and efficacy of vaccines.
- Logistical Barriers: In rural or conflict-affected areas, logistical barriers such as limited transportation and poor infrastructure can prevent vaccines from reaching those in need.
- Cost and Equity: High costs and unequal access to vaccines in lower-income countries remain significant barriers to global health equity. International collaborations are needed to ensure that vaccines are affordable and accessible to all populations.
The Future of Vaccines
The evolution of vaccines is far from over. As science continues to advance, new technologies and approaches are emerging that promise to make vaccines even more effective, easier to administer, and more widely accessible. Some key developments on the horizon include:
- Universal Vaccines: Researchers are working on universal vaccines for diseases like influenza, which would protect against multiple strains of the virus, reducing the need for yearly vaccinations.
- Oral and Nasal Vaccines: Efforts to develop vaccines that can be taken orally or through the nose aim to simplify administration and eliminate the need for injections.
- Vaccine Personalization: Advances in genomics and biotechnology may lead to personalized vaccines tailored to an individual’s genetic makeup, providing even greater protection against diseases.
Conclusion
The journey of vaccines, from their early development to the sophisticated technologies used today, has been a remarkable testament to human ingenuity. Vaccines have not only saved millions of lives but also prevented the spread of infectious diseases worldwide. However, challenges remain in ensuring equitable access, overcoming vaccine hesitancy, and managing complex distribution systems.
As research and technology continue to advance, vaccines will undoubtedly play an even larger role in safeguarding global health. By understanding the evolution of vaccines and the critical importance of their development and distribution, we can better appreciate the pivotal role they play in the fight against infectious diseases and the protection of public health worldwide.